The chemical resistance of PVC work boots stems directly from the stable, non-reactive molecular structure of Polyvinyl Chloride. This polymer is inherently resilient against a specific range of common industrial chemicals, preventing them from permeating or degrading the material upon contact and protecting the wearer's skin.
PVC offers reliable protection against many common substances like oils, greases, and certain acids because its polymer chains are difficult for these chemicals to break down. However, it is not a universal shield, and its effectiveness depends entirely on the specific chemical, its concentration, and the duration of exposure.

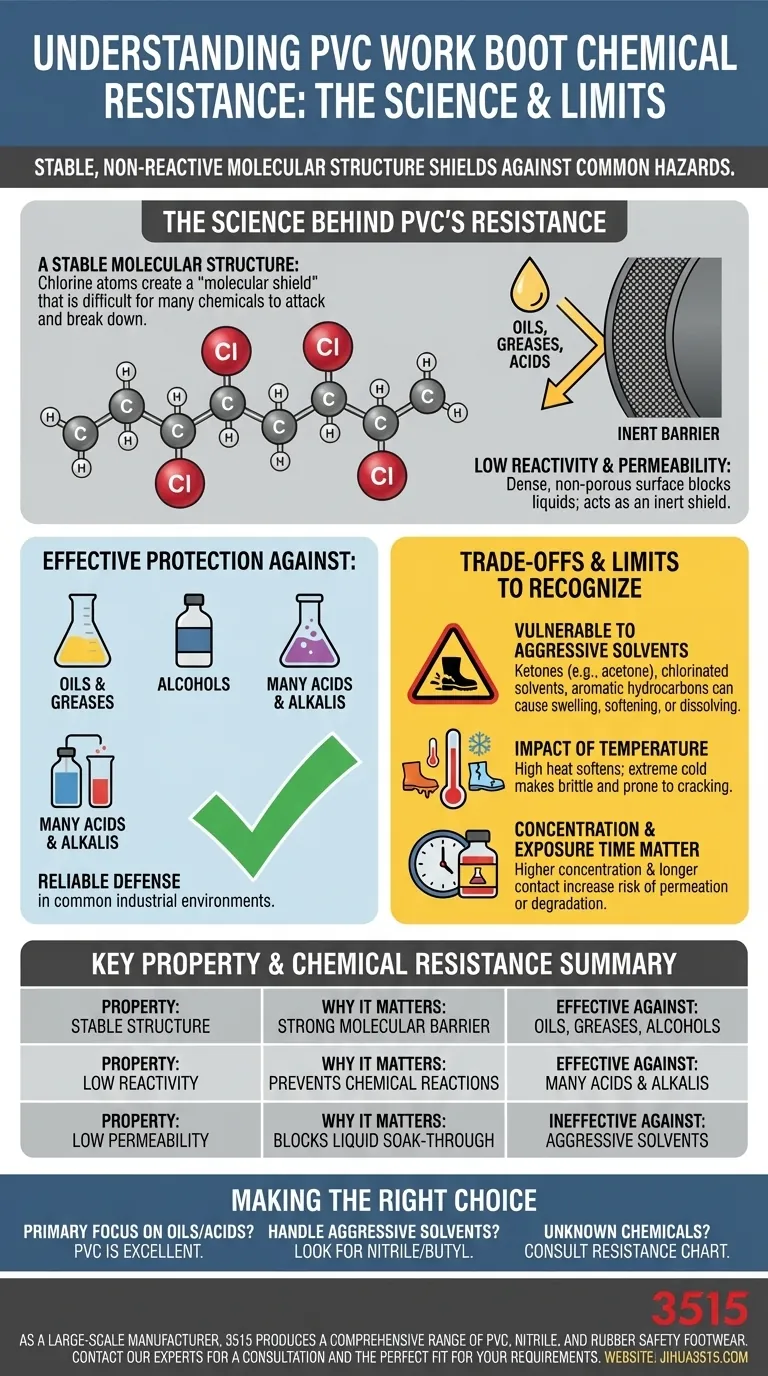
The Science Behind PVC's Resistance
To understand why PVC (Polyvinyl Chloride) works as a protective barrier, we need to look at its fundamental properties at a molecular level. The material isn't just a thick layer of plastic; its chemical composition is what provides the real defense.
A Stable Molecular Structure
PVC is a polymer, which means it's made of long, repeating chains of molecules. The presence of chlorine atoms in this structure is key. These atoms are large and strongly bonded to the carbon backbone, effectively creating a "molecular shield" that is difficult for many other chemicals to attack and break apart.
Low Reactivity and Permeability
This stable structure makes PVC inherently non-reactive with many common substances. Unlike materials that might absorb or react with chemicals, PVC acts as an inert barrier. Its dense, non-porous surface also has low permeability, which means it physically blocks liquids from soaking through to the foot.
Effective Against Common Substances
This molecular stability makes PVC highly effective against a range of chemicals frequently found in industrial, agricultural, and food processing environments. This includes excellent resistance to:
- Oils and greases
- Alcohols
- Many acids and alkalis
This is why PVC boots are a dependable choice in environments where these specific types of spills or splashes are a daily reality.
Understanding the Trade-offs: The Limits of PVC
No material is resistant to everything. Being an effective technical professional means understanding a material's weaknesses just as well as its strengths. PVC has clear limitations that are critical to recognize for safety.
Vulnerability to Specific Solvents
While resistant to many chemicals, PVC can be compromised by certain types of aggressive organic solvents. Chemicals like ketones (e.g., acetone), chlorinated solvents, and aromatic hydrocarbons can cause the PVC to swell, soften, or even dissolve over time, completely eliminating its protective qualities.
The Impact of Temperature
A material's chemical resistance can change with temperature. High temperatures can soften PVC, making it more susceptible to chemical attack. Conversely, extreme cold can make the material brittle and prone to cracking, which would create a physical path for chemicals to penetrate.
Concentration and Exposure Time Matter
Resistance is not an absolute guarantee. A boot might resist splashes of a diluted chemical but fail when exposed to the same chemical at a high concentration or for a prolonged period. The longer a chemical is in contact with the boot, the more time it has to potentially permeate or degrade the material.
Making the Right Choice for Your Environment
Choosing the right boot is a critical safety decision that requires matching the material to the specific hazards you face. A boot that provides perfect protection in one scenario can be dangerously inadequate in another.
- If your primary focus is protection from oils, greases, and common acids: PVC work boots offer an excellent and cost-effective solution.
- If you handle aggressive organic solvents like acetone, MEK, or toluene: You must look beyond standard PVC to more specialized materials like nitrile or butyl rubber boots.
- If your work involves a wide variety of unknown or mixed chemicals: You should consult a specific chemical resistance chart from the manufacturer before selecting any footwear.
Ultimately, understanding the specific chemical properties of your protective gear is the most critical step in ensuring your safety on the job.
Summary Table:
| Key Property | Why It Matters for Chemical Resistance |
|---|---|
| Stable Molecular Structure | Chlorine atoms create a strong barrier that is difficult for many chemicals to break down. |
| Low Reactivity | Acts as an inert shield, preventing chemical reactions with common industrial substances. |
| Low Permeability | Dense, non-porous surface blocks liquids from soaking through to the foot. |
| Effective Against | Oils, greases, alcohols, and many acids/alkalis. |
| Ineffective Against | Aggressive solvents like ketones (acetone) and aromatic hydrocarbons. |
Need the right chemical-resistant boots for your team? As a large-scale manufacturer, 3515 produces a comprehensive range of safety footwear for distributors, brand owners, and bulk clients. Our production capabilities encompass all types of PVC, nitrile, and rubber boots, ensuring you get the precise protection your workforce needs. Contact our experts today for a consultation and get the perfect fit for your safety requirements.
Visual Guide
Related Products
- Safety Footwear Wholesale Manufacturer for Custom OEM/ODM Production
- Premium Flame-Retardant Waterproof Safety Boots and Shoes
- Wholesale Safety Footwear Manufacturer for Bulk & Custom OEM Orders
- Heavy-Duty Waterproof Nubuck Safety Boots Safety Shoes for Bulk Supply
- Premium High-Cut Waterproof Safety Boots Manufacturing & Wholesale Solutions
People Also Ask
- What are the cost considerations when choosing between leather and rubber boots for hazmat situations?
- What are some key differences between traditional and modern engineer boots? Choose the Right Style for Your Needs
- What features should roofers look for in work boots? Essential Safety and Comfort for the Job
- What are logger or packer boots used for? Essential Gear for Uneven Terrain Work
- What are some reported drawbacks of this work boot from user reviews? Understand the Break-in Challenges
- What types of protection do composite toe work boots offer? Essential Safety for Modern Workplaces
- What is the purpose of providing a second pair of backup industrial-grade boots? Prevent Eczema & Improve Foot Health
- What are some additional features of quality composite toe work boots? Unlock Superior Durability & Comfort



















